Resident Editor: Kenneth Pettersen, MD
Faculty Editor: Christine Soran, MD
|
BOTTOM LINE ✔ Direct oral anticoagulants are increasingly first line therapy, though low molecular weight heparin or warfarin is preferred in special populations ✔ Choice of anticoagulant should be individualized to patient preferences and comorbidities – review contraindications and bleeding risk prior to initiating anticoagulation |
General Practices and Precautions
- Agent selection:
- Direct oral anticoagulants (DOACs) are increasingly first-line therapy given their non-inferior to superior thromboembolic efficacy and lower risk of intracranial hemorrhage.
- Warfarin remains first-line therapy in mechanical heart valves as well as ESRD.
- Low-molecular weight heparin (LMWH) is recommended for cancer-associated venous thromboembolism (VTE).
- Bleeding Risks:
- Avoid NSAIDs while on warfarin
- Consider starting a PPI in patients on dual antiplatelet therapy or aspirin + anticoagulant if: history of GI bleed, concomitant steroid use, dyspepsia or GERD symptoms, or 60+ years old (ACCF/ACG/AHA 2008 expert consensus)
- Various scoring systems have been created to estimate bleeding risk in patients with atrial fibrillation taking warfarin, including the ATRIA and HAS-BLED scores (see below). The general principles likely apply to DOACs as well as VTE.
- Duration of anticoagulation (general guidelines):
- Chronic atrial fibrillation and mechanical heart valves: lifelong
- Venous thromboembolism, provoked: 3 months
- Venous thromboembolism, unprovoked: ≥ 3 months versus lifelong depending on bleeding risks.
- Venous thromboembolism + active cancer: lifelong
- Mitral valvuloplasty, cardioversion: 3 weeks prior to procedure and 4 weeks afterwards
- Special considerations: Anticoagulation in patients with VTE/AFib and CAD (When to consider dual vs triple therapy):
- All anticoagulation and antiplatelet decisions should be made closely with the patient’s cardiologist.
- Adding DAPT onto anticoagulation increases bleeding risk 2-3 fold; increased risk also related to duration of therapy.
- Stable CAD without stents: anticoagulation alone (without antiplatelet therapy).
- ACS or new stent:
- Always consult with patient’s cardiologist
- Low-bleeding risk and new stent:
- 2014 AHA guidelines recommend triple therapy with anticoagulation + P2Y12 inhibitor + aspirin (1 month for BMS and 1-6 mo for DES) followed by dual therapy (anticoagulation + P2Y12 inhibitor) for 6-12 months.
- Of note, a 2017 RCT (RE-DUAL PCI) showed lower bleeding complications and non-inferior thromboembolic complications with dual therapy using dabigatran and a P2Y12 inhibitor compared to triple therapy. The WOEST trial similarly showed single antiplatelet therapy to have lower bleeding risk with no increased thrombotic risk. Updated AHA guidelines are not yet published.
- High bleeding risk and new stent OR ACS without stenting:
- Dual therapy is recommended with anticoagulation + P2Y12 inhibitor for 6-12 months.
- After 6-12 months, P2Y12 inhibitor is usually changed to aspirin.
Bleeding risk assessment in patients with in atrial fibrillation on warfarin
|
Risk Tool |
Risk Score Calculation |
Risk Categories (points) |
Annual Bleeding Rate |
|---|---|---|---|
|
HAS-BLED |
1 point for each: -Hypertension (SBP>160) -Abnormal renal function (dialysis, Cr>2.6) -Abnormal liver function (cirrhosis, bili >2x ULN, AST/ALT/AP >3x nl) -Stroke history -Bleeding history/predisposition -Labile INR -Elderly (age>65) -Drug/alcohol use (>8 drinks/wk)
|
Low (0) Intermediate (1-2) High (3+) |
1.1% 2.9% 3.7% |
|
ATRIA Risk Score |
-Anemia (3 points) -Severe renal disease (eGFR<30) (3 points) -Age>75 (2 points) -Prior bleed (1 point) -Hypertension (1 point) |
Low (0-3) Intermediate (4) |
0.8% 2.6% 5.8% |
Excellent app for evaluating stroke risk vs. bleeding risk: AnticoagEvaluator from the ACC
Direct Oral Anticoagulants (DOACs): Factor Xa and direct thrombin inhibitors
|
|
Factor Xa inhibitors |
Direct Thrombin inhibitor |
|
|---|---|---|---|
|
Agent |
RivaroXaban (Xarelto ®) |
ApiXaban (Eliquis ®) |
DabigaTran (Pradaxa ®) |
|
FDA approved for |
Non-valvular afib, VTE ppx, VTE tx |
Non-valvular afib, VTE tx |
Non-valvular afib, VTE tx |
|
Dosing for non-valvular afib |
20mg po daily (15mg po daily for CrCl 15-50) |
5mg po bid (2.5mg po bid if ≥ 2 of the following: Cr ≥ 1.5, age ≥ 80 yo, wt < 60 kg) |
150mg po bid (75mg po bid for CrCl 15-30) |
|
Dosing for VTE ppx (orthopedic) |
10mg po daily |
2.5mg po bid |
n/a |
|
Dosing for VTE tx |
15mg po bid x21 days, then 20mg po daily |
n/a |
150mg po bid after 50-10 days of parenteral anticoagulation |
|
Drug interactions |
CYP3A4/P-gp inhibitors |
CYP3A4/P-gp inhibitors |
P-gp inhibitors |
|
Efficacy in non-valvular Afib (compared to warfarin) |
Non-inferior |
Reduced all-cause mortality |
Trend towards reduction in all-cause mortality |
|
Bleeding risks in non-valvular Afib (compared to warfarin) |
Higher risk of GI bleed, lower ICH |
Lower risk of major bleeding (ICH and GI bleed) |
Higher risk of GI bleed, lower risk of ICH |
|
Target-specific antidote(s) |
Under investigation; KCentra used in practice |
Under investigation; KCentra used in practice |
Idarucicumab |
|
Special considerations |
Should be taken with largest meal of the day |
|
Dyspepsia present in up to 10% of patients |
- Contraindications: mechanical valve, low CrCl (<15-30)
- No INR monitoring, fewer drug interactions, activity independent of vitamin K
- Fast onset so no need for bridging
- Less reliable bleeding antidotes compared to warfarin
- No reliable method of monitoring anticoagulant effect; agents may inconsistently elevate PT or PTT but these tests cannot be used for monitoring
- Not well-studied in pregnancy, extremes of weight (<110 lbs or >250 lbs), cancer-associated VTE, or mechanical heart valves
- No standard recommendations for follow-up, however, if starting a new oral anticoagulant, reasonable to:
- Obtain baseline CBC, Cr, PTT/PT, LFTs
- Monitor for adherence, adverse effects, bleeding/stroke at: 1 week, 2 weeks, 1 month, and 3 months
- Monitor CBC and Cr at least twice a year
- Surgery:
- Overall, guidelines are still in progress, but generally principles are similar to warfarin (see above) with a tendency towards no bridging given predictable pharmacokinetics.
- Timing of pre-operative discontinuation of DOACs varies depending on agent, thrombosis risk, surgical bleeding risk, and CrCl (range 2 to 5 days).
- Post-surgical resumption of DOACs is typically 1-2 days following a low bleeding risk procedure and 2-3 days after a major bleeding risk procedure.
Warfarin
Initiation of warfarin
- To calculate patient-specific initiation and maintenance dose, go to: www.warfarindosing.org. A common starting dose is 5mg po daily. If more rapid anticoagulation is desired, can give 1.5x the maintenance dose x 2 days.
- Patients with acute VTE: overlap parenteral anticoagulant (LMWH or heparin) with warfarin for at least 5 days, and then continue until INR is therapeutic. Can start warfarin on day 1 of LMWH or heparin.
- Alternating day doses of warfarin are commonly used: example, to achieve a total weekly warfarin dose of 30mg, can prescribe: 2.5mg on Mondays/Fridays and 5mg all other days.
- Lower starting doses of warfarin may be needed for: women, low body weight, Asian race, poor nutritional status, age > 75, liver disease, increased bleeding risk, hyperthyroidism, certain medications (amiodarone, statins, azoles).
- Higher starting doses of warfarin may be needed for: weight >85 kg, African-Americans, hypothyroidism, certain medications (rifampin, some anti-epileptics)
- Counsel patients to maintain stable vitamin K intake (consistent dietary intake of leafy green vegetables like kale, collards, spinach).
Frequency of INR checks
- Once warfarin is initiated, check INRs every 3-4 days until 2 consecutive INRs are therapeutic. Then check weekly INRs until 2 consecutive INRs are therapeutic on a stable warfarin dose. Once warfarin dose is stable, obtain regular INR checks q4-6 weeks (or more frequently as needed for acute illness, medication changes, out of range INRs).
- If INR and warfarin doses are very stable for > 6 months, can consider testing q3 months.
- If INR is out of range, adjust total weekly dose by 5 – 20% (depending on degree to which INR is out of range) and recheck INR within ~1 week (or sooner if INR is >5). Example: if INR = 3.5 on 20mg per week of warfarin, reduce dose by 20% = 16mg weekly.
- Hospitalization often results in out of range INRs. Recommend INR check within 3-4 days of hospital discharge.
Management of supratherapeutic INR:
- For patients with major bleeding, refer to higher level of care (refer to UCSF Hospitalist Handbook)
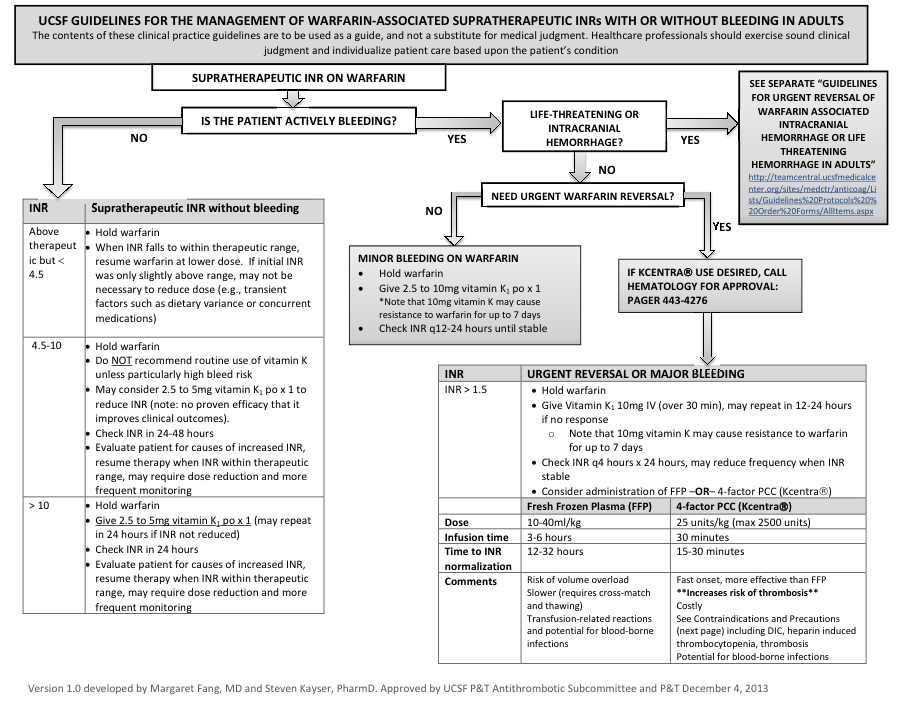
Figure by Margaret Fang, MD, and Steven Kayser, PharmD, reproduced with permission of authors.
Management of warfarin in perioperative setting
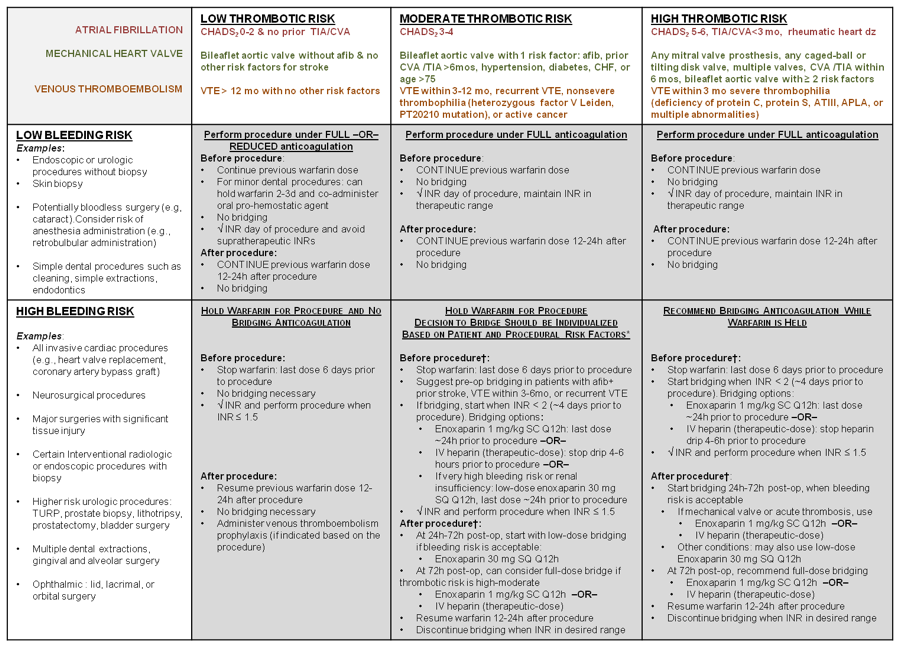
Table by Margaret Fang, MD, and Steven Kayser, PharmD, reproduced with permission of authors.
Precautions with warfarin - Many drugs can increase INR: azoles, amiodarone, statins, various antibiotics. Perform interaction check prior to prescribing, or monitor INR more frequently during initiation
- Drugs which can decrease INR: rifampin, some anti-epileptics
- Teratogenicity: warfarin is a teratogen and should not be given if there is any suspicion for pregnancy (perform pregnancy test). Heparin and LMWH are thought to be safe in pregnancy but should be managed by a specialist.
Complications of anticoagulation
- Bleeding: Bleeding risk should be assessed at the time of considering initiation of anticoagulation and at least annually in patients with indication for long term therapy. Intracranial hemorrhage (ICH) occurs in < 1% of all patients but risk is elevated in patients 75 and older, those with hypertension, and renal insufficiency.
- Hypercoagulability: Patients are hypercoagulable during the first 24-36 hours of warfarin therapy. For people with acute thrombosis or Protein C or S deficiency (skin necrosis risk), recommend concomitant parenteral anticoagulation with LMWH.
- Reversal guidelines at UCSF: see above flowchart
Chronic LMWH therapy
- May be more effective for cancer-related VTE.
- Avoid in renal insufficiency (CrCl < 30)
- Precautions: occasionally patients on heparin will get hypersensitivity reactions, LFT abnormalities, and long-term use can predispose to osteoporosis.
- If on therapy, check renal function at least yearly. Check platelets at least q6 months to monitor for HIT. If HIT, transition to fondaparinux.
- May be reasonable to check Factor Xa levels in selected patients (such as extremes of body weight, renal insufficiency, pregnancy)
- Dosing: 1 mg/kg SQ BID or 1.5 mg/kg daily in patients w/o cancer or large clot
References:
Ageno W, et al. Oral anticoagulant therapy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(Suppl):e44S-88S.
Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus documentation on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008;118:1894-1909.
Cannon CP, et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. NEJM. 2017 Oct 19;377(16):1513-1524.
Fang MC, Go AS, Chang Y, et al.. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011 July 19; 58(4): 395–401.
Fang MC, Kayser S. UCSF Anticoagulation Guidelines. Available at: http://teamcentral.ucsfmedicalcenter.org/sites/medctr/anticoag/Lists/Guidelines%20Protocols%20%20Order%20Forms/AllItems.aspx. Accessed June 28, 2014 (from UCSF vpn only).
Holbrook A, Schulman S, Witt DM, et al. Evidence-Based Management of Anticoagulant Therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2): e44S-88S.
January CT, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. Circulation. 2014 Dec 2;130(23):2071-104.
Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. CHEST 2016; 149(2):315-352.
Piccini JP, Jones WS. Triple Therapy for Atrial Fibrillation after PCI. NEJM. 2017 Oct 19;377(16):1580-1582.
Pisters R, Lan DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-100.